The current activities of the heterogeneous catalysis lab are focused on understanding catalytic process for screening novel catalytic materials as well as implementing the findings in surface science studies in high surface area catalysts. .We focus on synthesis, characterization and testing of high surface area catalysts. We continuously collaborate with the theory group CatTheory at DTU Physics to explore new catalytic materials. Our approach involves either a circular or a zigzag pattern of complimenting the activities at different steps, namely, theory, model system, characterization and synthesis and testing in order to move closer to finding the best catalyst. The researches that are currently ongoing are as follow:
- Fixation of CO2 with H2 for fuel production: The research performed on this topic is related to production of methanol and other higher alcohols at low temperature and pressure conditions by fixing CO2 with H2. We have invented a Ga based intermetallic catalysts that shows comparable activities to the industrially used Cu/Zn/Al2O3 [1–3]. Fundamental understanding of the Cu/Zn/Al2O3 system is investigated in collaboration with Halder Topsøe A/S [4, 5] Pd2Ga
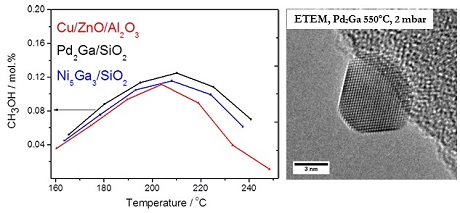
The figure is showing that the activity of Ni5Ga3/SiO2 and Pd2Ga is comparable to the industrially used Cu/ZnO/Al2O3. Reaction condition: 25% CO2, 25% H2, rest inert at atmospheric pressure[3]
- Hydrogen generation from ammonia: Ammonia is a high density hydrogen carrier and a zero-carbon fuel but in order to use ammonia as fuel for low temperature fuel cells, it needs to be decomposed through a high temperature catalytic process. The best know catalysts for this process is ruthenium which is a rare metal. We are approaching the reaction from the fundamental point of view in order to find abundant metal catalysts. We have come up with an alloy catalyst based on FeNi that shows comparable activity with hughly optimized Ru/al2O3 catalysts for ammonia decomposition[6].
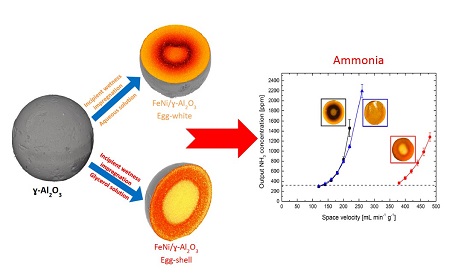
The figure shows that specially synthesized egg shell type catalyst gives much higher performance that uniformly distributed FeNi on alumina pelletsf [7]
Bottom up design of catalyst:
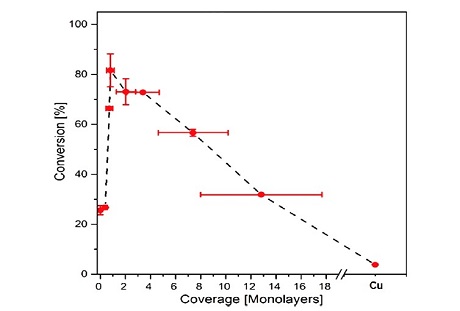
We also focus on industrially relevant catalyst design based on insight from surface science studies and theory. One such study has been published in Angewandte Chemie about ammonia oxidation on a Cu/Ru catalyst. The figure shows the effect of copper overlayer thickness on the activity[8].
- Selective oxidation of ammonia in H2: removal of trace ammonia from mixture containing up to 75% of H2 is the focus of the research. One of the possible ways to achieve the trace ammonia removal is to selectively oxidize the ammonia present. But because H2 is so much easier to oxidize compared to ammonia, one needs an extremely selective catalysts for the ammonia oxidation, we have invented a set of catalysts of Fe, Co, Re and Cr on TiO2 for the selective oxidation of trace ammonia in H2. The present work in this area deals with understanding the reaction on the catalysts as well as improving activity and stability of the catalysts.