Electrochemical Halide Coupling to Hydrocarbons
Project period: 2020-2023
Grant DK kr. 3,9 mio
With cheapening electricity from renewables such as wind and solar, the chemicals industry is currently in the initial stages of an evolution towards sustainable, electrochemically derived products. While reductive reactions such as H2 evolution and CO2 reduction are becoming well developed, they still use the valueless O2 evolution as the oxidative counter reaction. Electrochemical halogenation of hydrocarbons is an alternative oxidative process that could reduce the costs of high value products such as Teflon, polyvinyl chloride (PVC), and fire-retardant materials.
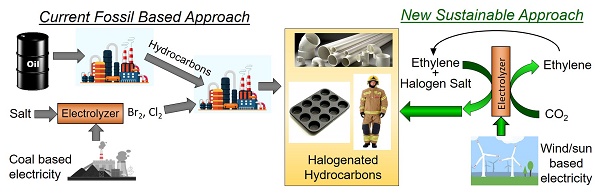
Figure 1:Current & future approach toward HH products.
The current approach to most hydrocarbon halogenations (HH) involves halogen gas production through electrolysis followed by an exothermic reaction of these gases with the hydrocarbons (Figure 1). Rather than use a multi-step process that involves a poisonous gas and produces waste heat, this project will focus on a single step electrochemical process that can operate at lower potentials than halogenated gas production, thus mitigating safety concerns and maximizing energy efficiency. The largest barrier to implementing this process is the lack of an efficient catalyst. Thus, this project’s objective will be to develop fundamental understanding relating to electrocatalytic halogenation of hydrocarbons, and to relate this to the development of new, optimized catalysts. Current electrohalogenation processes marginalizes the catalyst and focuses on redox couples attacking organics in solution or inefficient radical based mechanisms that takes place in organic solvents. This project will focus on surface-based catalysis, which allows for greater efficiency, higher rates, and greater control over selectivity. The vast knowledge base related to the electrochemically coupling of C, H, and O will be used as a basis on which to build fundamental knowledge relating to carbon-halogen (C-X) coupling, which can help broaden the scope and potential of electrocatalysis.
An optimal catalyst needs efficient 1) halide adsorption, 2) hydrocarbon adsorption, and 3) halide-hydrocarbon coupling. The goal will be to find a catalyst that has a moderate binding energy to both the hydrocarbon and the halide (X), thus allowing for halide-hydrocarbon coupling. The thermodynamic driving force favoring C-X coupling over X2 gas evolution is on the order of 0.6-0.8 V, thus providing a large buffer to find a selective catalyst. The reaction will operate in an environmentally friendly aqueous environment, thus entailing hydroxide adsorption will also need to be considered. Using this approach allows for the possibility to design catalysts geared towards valuable oxygenated species such as chloroethanol and chloroacetic acid, thus both increasing the commercial potential, as well as broadening the scientific scope.