VALence Band Tuning of ElectroCATalyst for the CO2 Reduction Reaction
Project period 2019 -2021
Grant: DK 1.55 Mio
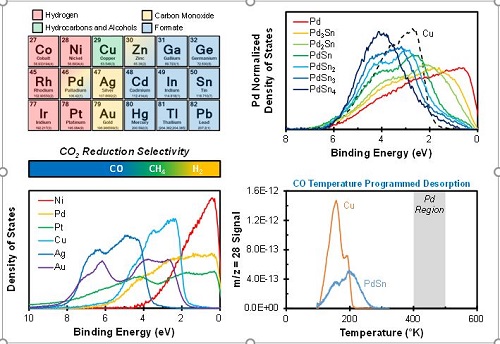
The electrochemical reduction of CO2 enables carbon-neutral fuels and chemicals to be produced using intermittent renewable electricity. However, monometallic Cu is the only electrocatalyst capable of driving this reaction with a technologically relevant selectivity for hydrocarbons and alcohols and its voltage efficiency is not sufficient to make the process economically viable.1 Unfortunately, electrocatalyst discovery efforts over the past 30 years have failed to identify alternative electrocatalysts for this reaction, let alone electrocatalysts with superior activity to monometallic Cu. The rate-determining step in the reduction of CO2 to hydrocarbons and alcohols has been identified to be the reduction of a CO intermediate. Thus, the unique selectivity observed over monometallic Cu has been attributed to its moderate CO adsorption energy, which is unique among transition metals.2 The CO adsorption energy of transition metals is determined by the d-band portion of their valence band density of states.3 Thus, superior electrocatalysts to monometallic Cu can likely be discovered through directed systematic electronic structure tuning. The electronic structure of transition metals can be systematically modified through the formation of strong heteronuclear bonds with electronically dissimilar metals. The CO2RR VALCAT project aims to leverage this approach to:
- Identify the electrocatalyst properties required to catalyze CO reduction. The surface reactivity of transition metals is determined by the d-band portion of their valence band density of states. Cu possess d-band valence states in a narrow binding energy regime centered at ~3 eV, unlike any other transition metal. Thus, the unique ability of Cu to catalyze CO reduction is likely a result of this unique valence electronic structure. We will explore this hypothesis by synthesizing Cu-free intermetallic alloys with nearly identical valence electronic structures and measuring their electrocatalytic activity for CO reduction.
- Demonstrate the impact of systematic electronic structure modifications on the CO reduction activity of transition metals. The d-band portion of the valence band density of states of a transition metal can be systematically modified via the formation of a strong heteronuclear bonds with electronically dissimilar metals, such as those found in intermetallic alloys. We will investigate the extent to which surface reactivity can be tuned through systematic d-band modifications and will demonstrate the impact such modifications have on catalytic activity and selectivity.
- Discover novel electrocatalysts for CO reduction with superior activity to Cu. The strong heteronuclear bonds characteristic of intermetallic alloys result in periodic structural order. Co-locating different metal sites results in the formation of catalytically active motifs that can enhance the stability of transition states though bidentate binding to metal sites with disparate chemical reactivity, as are present in many enzymes.
References
- Hori, Y.; Kikuchi, K.; Suzuki, S. Chem. Lett. 14 (1985).
- Peterson, A. A.; Nørskov, J. K. J. Phys. Chem. Lett. 3 (2012).
- Nilsson, A.; Pettersson, L. G. M.; Hammer, B.; Bligaard, T.; Christensen, C. H.; Nørskov, J. K. Catal. Lett. 100 (2005).
- Bligaard, T.; Nørskov, J. K. Electrochimica Acta 52 (2007).
- Iwasa, I.; Masuda, S.; Ogawa, N.; Takezawa, N. Appl. Catal. A 125 (1995).