Project period: 2010-2014
H2-assisted ammonia SCR project is funded by The Danish Council for Strategic Research and aims at developing a competitive system for removal of NOx from diesel engines that is partly based on H2-assisted selective catalytic reduction of NOx with ammonia.
The work is based on the observation that a very high low temperature (150 – 250 °C) activity SCR is obtained over an Ag/Al2O3 catalyst if hydrogen is dosed together with ammonia. If such a catalyst is combined with known SCR catalysts (e.g. Fe-BEA) a high activity can be obtained in the whole relevant temperature interval. The combination with such a medium temperature range (250 – 400 °C) catalyst also limits the need for H2-dosing to the lowest temperatures, thus making the system more energy-efficient.
Challenges
Low temperature activity of the SCR catalyst (i.e. from 150 °C)
Sulphur tolerance (ca. 1 ppm SO2 present in exhaust)
Fast and accurate dosing of NH3 and H2
Fast and accurate kinetic modelMotivation
The market of the light-duty diesel-powered cars has grown during the last decade in Europe and also in the US. The reason for this is significantly less fuel consumption of diesel engines related to gasoline, which means better fuel economy and reduced CO2 emissions. In many countries there are also government incentives such as tax reduction for low CO2 emitting vehicles. The down side of diesel engines is the higher emission of harmful nitrogen oxides (NOx) and soot (Particulate matter, PM), as compared to gasoline vehicles with three-way catalysts (TWC). A distinctive feature of the diesel engine is that it is operating under non-stoichiometric air/fuel ratio with excess of air, so called lean conditions. TWCs used for gasoline cars cannot reduce NOx from the exhaust containing excess of oxygen and therefore other catalysts has to be used for removal of NOx from diesel exhausts.
Today there are two aftertreatment technologies available for lowering NOx emission from diesel-powered vehicles: selective catalytic reduction (SCR) with ammonia or urea (AdBlue) and lean NOx traps. SCR is the most robust and energy efficient of the two (energy efficiency = improved fuel economy). Urea-SCR is widely used for heavy-duty trucks to comply with Euro V. It is also implemented in a few light-duty vehicles. The stricter US tier 2, bin 5 (2009) and the up-coming and Euro 6 (2014) could see the demand for SCR on light-duty vehicles increase.
Light-duty diesel engines have exhausts with low temperature. This is tough for catalyst producers since the low temperature activity of SCR catalyst still is a challenge.
Results
Optimization of Ag/Al2O3 catalyst: A high SBET and higher Ag loading gave a high sulphur tolerance and activity. It was believed that the high SBET is needed to give a higher NH3 adsorption capacity, necessary for the SCR reaction.
A higher Ag loading gives more Ag sites and probably a favorable Ag dispersion. Testing with sulphur gave an increased activity of the catalysts. Testing of monolithic catalysts showed a similar activity enhancement after a few standard test cycles. A change in the dispersion or state of Ag can be possible reasons for the activation seen and the activation was believed to be related to Ag and not the alumina.
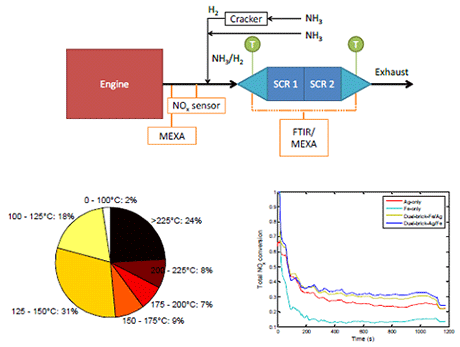
Full scale engine testing: Small-scale laboratory testing showed that it was preferred to have Ag/Al2O3 either upstream or as the outer layer of Fe-BEA. This was attributed to complete NH3 oxidation over Fe-BEA giving a deficit of NH3 over the Ag/Al2O3 if it was placed downstream or as the inner layer. Full-scale engine testing, on the other hand, showed the opposite for a dual-brick layout. High NO2 concentrations are believed to give fast-SCR over the Fe-BEA when it was placed upstream of the Ag/Al2O3. The activity of the combined catalyst layouts were higher than the activity for individual catalysts when less or no H2 was co-fed in the small-scale case showing that there were synergistic effects by combining them. The dual-layer layout showed the best performance which is believed to be attributed the short diffusion distance between the layers allowing diffusion of reaction intermediates between them.
Ag/Al2O3 only and the combined Ag/Al2O3 – Fe-BEA systems were active during the transient NEDC. The NOx conversions were not very high which is related to the very low temperature of the NEDC and the lower than expected activity of the Ag/Al2O3 catalyst seen in stationary testing. The most interesting result was that the catalyst systems showed NOx conversion already from the start of the cycle, before any NH3 or H2 was dosed. NOx storage over the Ag/Al2O3 was believed to be the most likely explanation for this. The NOx conversion could be enhanced by dosing of NH3 and H2 at temperatures lower than 150°C that was used as standard starting temperature of dosing. However, dosing too early inhibited the NOx conversion.